 Xingaonai
Xingaonai
Silicon dioxide, with the chemical formula SiO₂, is an important inorganic compound. As a typical representative of acidic oxides, its unique physical and chemical properties and diverse existence forms make it an indispensable part of industry, scientific research and daily life. Xingaonai Heavy Industry will systematically explain the characteristics of silicon dioxide and its diverse uses.

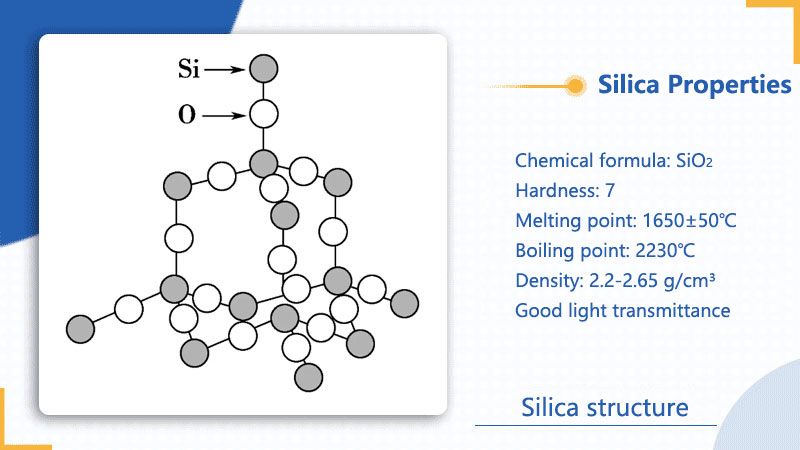
Physical properties
Silicon dioxide has high hardness (Mohs hardness 7), a melting point of 1650±50℃, a boiling point of 2230℃, is solid at room temperature, and has a density of about 2.2-2.65 g/cm³. Its crystal structure is a three-dimensional network in space, and each silicon atom forms a tetrahedron with four oxygen atoms, giving it high hardness, high melting point and good mechanical strength. It is insoluble in water and most acids, but can react with hydrofluoric acid (HF) to form hexafluorosilicic acid (H₂SiF₆), and is soluble in hot concentrated phosphoric acid and molten alkalis. Pure silicon dioxide crystals are colorless and transparent, with high refractive index and good light transmittance, suitable for optical applications.
Chemical properties
Silicon dioxide can react with alkaline oxides (such as calcium oxide) at high temperatures to form silicates, and react with alkalis (such as NaOH) to form sodium silicate (water glass), but because it is insoluble in water, it cannot react directly with water to form silicic acid. Silicon dioxide is also the only acidic oxide that can react with hydrofluoric acid, and this property is used in glass engraving and etching processes. In addition, silicon dioxide is non-toxic, but long-term inhalation of silicon dioxide dust may cause silicosis, so occupational protection should be paid attention to.
Silicon dioxide has a wide range of uses, involving multiple industrial and scientific research fields.
Glass manufacturing industry
Silicon dioxide is a key raw material for the manufacture of flat glass, glass products and quartz glass. Its superior high temperature resistance makes it an ideal material for the manufacture of chemical instruments, especially in quartz glass, which has an extremely low expansion coefficient and excellent acid resistance.
Electronics industry
Silicon dioxide also plays an important role. It is the basic material for the production of semiconductor devices and optical fibers, which are essential for modern communication technology. In addition, silica is also used to manufacture optical instruments and crafts, using its high hardness and optical transparency to make precise and durable products.
Construction field
Silica is also widely used in the construction industry, not only for the manufacture of ceramics and refractory materials, but also as a concrete additive to improve the strength and durability of materials. Various forms of silica, such as quartz sand, are widely used as raw materials for foundry sand and glass fiber, enhancing the functionality and aesthetics of building materials.
Plastic and rubber industry
As a highly efficient filler, silica can significantly improve the mechanical properties of materials, such as strength, toughness and wear resistance. Its application in coatings is also increasing, which can improve the weather resistance and washability of coatings, making the coatings more solid and beautiful.
Cosmetics and food
Silica also plays an important role in the cosmetics and food industries. As an oil absorbent and anti-caking agent in cosmetics, it helps to improve the stability and user experience of the product. In the food field, silica is used as an anti-caking agent to ensure the fluidity and dryness of powdered foods in compliance with food safety standards.

Although silica has a wide range of uses, its health hazards should also be noted during use. Silica itself is non-toxic, but long-term inhalation of silica dust may lead to serious lung diseases such as silicosis. Therefore, in the environment where silica is produced and used, effective protective measures must be taken to ensure the health and safety of workers.
In short, silica has shown irreplaceable value from traditional industries to high-tech fields due to its stable physical and chemical properties. With the advancement of nanotechnology and materials science, its application range will continue to expand, providing more possibilities for industry, technology and life in human society. In the application process, it is necessary to balance its excellent performance with occupational safety and achieve sustainable development through technical optimization.
Superior: No content!
Abajo: No content!
Nuestros productos se han exportado a más de 170 países de África, Asia, América del Sur, Europa, etc. Estamos aquí para ofrecerle mejores productos y servicios.
Copyright © 2024-2030 Xingaonai Group Todos los derechos reservados. Sitemap