
 Xingaonai
Xingaonai
As a versatile carbonate mineral, dolomite is mainly composed of calcium, magnesium and carbonate, and is famous for its unique properties and diverse applications. As a versatile mineral, dolomite not only reveals the history of sedimentation and metamorphism in geological research, but also plays a key role in industry, environmental protection, energy and other fields. With the advancement of science and technology, its application prospects will continue to expand and become an important resource for materials science and sustainable development.

Xingaonai Heavy Industry will lead you to explore five important topics of dolomite in depth: What is dolomite? Where is the Dolomite Mountain? How is dolomite formed? What are the characteristics of dolomite? What are the uses of dolomite? Only by understanding these key issues can you know the importance of dolomite more deeply.
Dolomite is an important carbonate mineral with the chemical formula CaMg(CO₃)₂, which is composed of calcium, magnesium and carbonate. Its crystal structure belongs to the trigonal system, often in the form of rhombohedron or block aggregate, with a curved crystal face like a saddle and a glassy luster. Pure dolomite is white, but can be gray, green, pink, etc. due to impurities. It has a hardness of 3.5-4 and a density of 2.8-2.9.
An important characteristic of dolomite is that it will bubble and hiss when it encounters hydrochloric acid, but this process is slower than limestone. This unique reaction is attributed to the presence of magnesium in dolomite, which has a lower reactivity with acid than calcium.

The Dolomites, also known as the Dolomites, are located in the Alps in northeastern Italy and are part of the Southern Limestone Alps. The mountains are geographically close to Venice and Milan, and major towns such as Bolzano and Cortina d'Ampezzo are within its range, the latter being a famous ski resort. The Dolomites were listed as a World Natural Heritage Site by UNESCO in 2009 for their unique pale pink limestone geological structure and magnificent landscape.

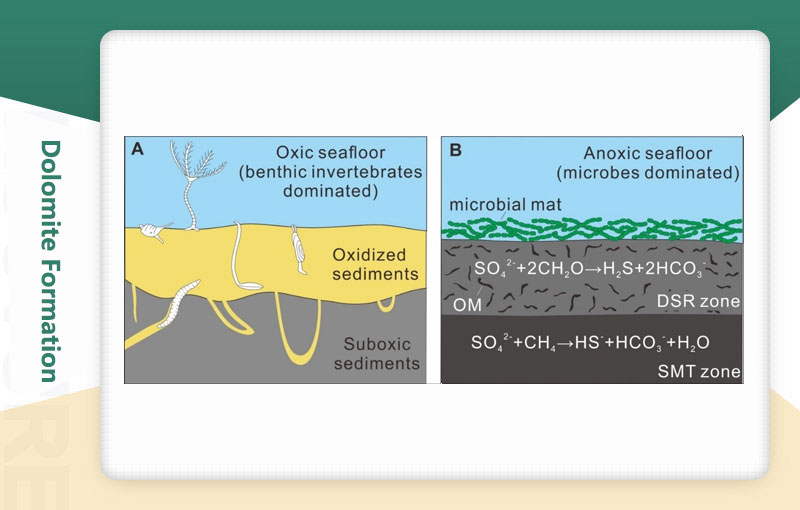
Dolomite is formed mainly through dolomitization, which is the process of magnesium ions (Mg²⁺) replacing calcium ions (Ca²⁺) in limestone (mainly calcite CaCO3) to generate dolomite. This process requires two key conditions: 1) the presence of an aqueous solution rich in magnesium ions; 2) a geological environment that promotes the full reaction of aqueous solution and limestone. Organisms cannot directly secrete dolomite, so its formation is almost entirely dependent on geochemical metasomatism.
The formation of dolomite is the result of a complex exchange of magnesium and calcium ions between geological fluids and carbonate rocks in geological history. From evaporation and concentration in the supratidal zone to hydrothermal transformation in the deep, different mechanisms have jointly constructed the diversity of dolomite.
Chemical characteristics
Chemical composition: Dolomite is composed of calcium (Ca), magnesium (Mg) and carbonate (CO₃²⁻), and the ratio of calcium to magnesium is usually 1:1. Its crystal structure is similar to that of calcite (CaCO₃), but the presence of magnesium ions makes it more stable in structure.
Crystal form: It belongs to the trigonal system, often presents rhombohedral or columnar crystals, and the crystal faces are often bent into a saddle shape. The phenomenon of polysynthetic twinning is common, forming blocky or granular aggregates.
Physical properties
Color and luster: Pure dolomite is white, but it can be gray-green, gray-yellow, pink and other tones when it contains impurities such as iron and manganese. The surface has a glassy luster, and may show a pearly luster after partial weathering.
Hardness and density: Mohs hardness is 3.5-4, slightly higher than calcite; specific gravity is 2.8-2.9, the texture is brittle and hard, and the rhombohedral cleavage is complete.
Acid reaction characteristics: It reacts slowly when exposed to cold dilute hydrochloric acid, and it needs to be heated or ground into powder before it can bubble significantly. This characteristic is an important sign to distinguish dolomite from calcite.
Optical properties: Some dolomites can emit orange-red light under cathode rays, and have a certain fluorescence effect.

Refractory materials: Dolomite is calcined at high temperature to form a material with high refractoriness. It is often used to make refractory products such as magnesia-calcium bricks and magnesia-calcium carbon bricks, and is used for the lining of high-temperature equipment such as steel smelting furnaces and cement kilns.
Cement production: It participates in the production of cement clinker as a raw material to improve the strength and durability of cement.
Glass manufacturing: It is used as a flux to reduce the melting point of glass and improve the transparency and stability of glass.
Ceramic industry: It is used for blanks and glazes to improve the mechanical properties and heat resistance of ceramics, and can also be used to produce new structural ceramics.
Architectural decoration: The processed dolomite stone is used for wall and floor decoration, or as an additive for concrete and mortar to enhance the hardness and corrosion resistance of building materials.
Steelmaking flux: It is used as a slag-making agent in the steelmaking process to adjust the composition and fluidity of slag, reduce the melting point, and improve the quality of steel.
Desulfurizer: Magnesium vapor is generated by reducing dolomite with ferrosilicon, which is used for desulfurization of molten iron to reduce sulfur impurities.
Chemical raw materials: used to produce basic chemicals such as calcium magnesium phosphate fertilizer, magnesium sulfate, and soda ash (sodium carbonate). Magnesium sulfate is widely used in the pharmaceutical, papermaking, printing and dyeing industries.
Agricultural applications: as a soil conditioner, it provides trace elements such as calcium and magnesium to improve the structure of acidic soil and enhance fertility; or mixed with potassium feldspar to produce potassium calcium fertilizer.
Wastewater treatment: finely ground dolomite powder (such as 200 mesh) as an adsorbent can efficiently adsorb heavy metals, phosphorus, boron and pollutants in printing and dyeing wastewater, with low cost and no secondary pollution.
Plastic and rubber fillers: ground dolomite as a filler to improve the hardness, wear resistance and anti-aging properties of products and reduce costs.
Paint industry: improves the weather resistance, oil absorption and scrubbing resistance of paints, and is often used in exterior wall paints and paints.
Papermaking industry: as a filler to improve the whiteness and smoothness of paper.
Energy field: as a catalyst carrier, it helps convert low-energy-density biomass into bio-oil and optimizes energy utilization.

If you want to know more about dolomite crushing production line and other information, you can consult customer service online or call +8617761642222. Xingaonai Heavy Industry will arrange senior engineers to answer your questions as soon as possible!

Superior: No content!
Abajo: No content!
Nuestros productos se han exportado a más de 170 países de África, Asia, América del Sur, Europa, etc. Estamos aquí para ofrecerle mejores productos y servicios.
Copyright © 2024-2030 Xingaonai Group Todos los derechos reservados. Sitemap